Published on 18 October 2021
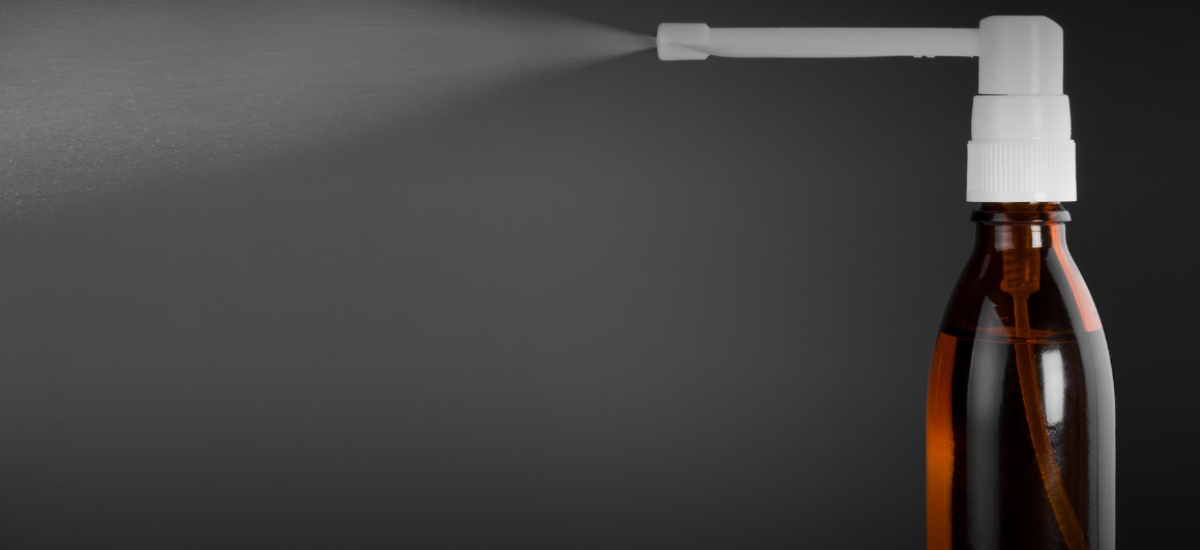
Two widely-used therapies – oral hydroxychloroquine and povidone-iodine throat spray – have been found to significantly reduce the risk of COVID-19 infection, according to a study by a team of NUHS clinician-scientists.
Conducted on 3,037 healthy migrant workers quarantined in Tuas South Dormitory last year, the trial randomly assigned participants to receive a six-week preventive course of one of five therapies: oral hydroxychloroquine, povidone-iodine throat spray, oral ivermectin, oral zinc/ vitamin C combination, and oral vitamin C.
Vitamin C was used as a comparator medication due to its popularity as a remedy during the pandemic, the scientists said.
The results showed that hydroxychloroquine and the throat spray reduced infection rates by 21 and 24 per cent respectively, when compared against a vitamin C regimen.
In contrast, the zinc/ vitamin C combination and ivermectin did not produce any significant risk reduction, the study found, although men who received ivermectin had fewer symptomatic infections.
No deaths or pneumonia resulting in hospitalisation occurred.
A/Prof Raymond Seet, the study’s lead author, noted that the study is the first large-scale trial to focus solely on the preventive properties of these therapies for patients who have not yet been exposed to the virus, rather than their ability to treat COVID-19.
It is also novel in that it includes topical therapy in the form of povidone-iodine spray, added A/Prof Seet, who is also Senior Consultant at NUH’s Division of Neurology. The spray was selected for its rapid virucidal properties, to shield the mucosal barrier in the throat and kill invading viruses.
“These existing drugs could be used to complement existing safety measures in settings where transmission is high while awaiting roll-out of a vaccine,” he added.
As they have to be used continuously, one possible strategy would be to have individuals take the medication when they enter a high-risk setting – such as a cruise ship or a nursing home – and then stop taking it once they leave the setting, said Prof Paul Tambyah, one of the study’s co-authors and Senior Consultant at NUH’s Division of Infectious Diseases.
“But if the whole community is high-risk, then you have to keep taking it until you bring the numbers down and it becomes a low-risk setting – or, ideally, you vaccinate,” he added.
The team highlighted that the repurposing of existing drugs is an important global strategy against COVID-19 as no medications have been shown to be effective in COVID-19 prevention so far.
This is especially since both hydroxychloroquine and povidone-iodine spray are easily available drugs with well-known safety profiles.
“This can represent a viable preventive strategy for individuals living in a closed and high-exposure setting, especially in areas and countries where COVID-19 vaccination is not available or widespread,” explained A/Prof Seet.
A high-profile drug
Hydroxychloroquine, an anti-malaria drug which is also used to treat auto-immune diseases like arthritis, has been the subject of intense scrutiny ever since it was touted as a ‘miracle’ cure by then-US President Donald Trump.
A lack of significant benefit in treating hospitalised COVID-19 patients, as well as a now-retracted paper in a leading medical journal that linked it to higher rates of death and heart-related complications in COVID-19 patients, led to its eventual decline in popularity.
In June 2020, the World Health Organisation announced that the hydroxychloroquine arm of the Solidarity Trial to find an effective COVID-19 treatment was being stopped.
“With hydroxychloroquine, at high doses, people were concerned about it because of its cardiac side effects,” said Prof Tambyah, explaining that it belongs to a family of therapies that is used to treat cardiac disorders.
“But with the dose we use, which is actually lower than what is being used for arthritis…we’ve shown that it is safe.”
The team noted that additional safety measures were taken in the hydroxychloroquine arm. For instance, participants had to undergo an echocardiogram and were excluded from the trial if they displayed any abnormalities, such as arrhythmia.
A community effort

Despite the controversy surrounding the drug, A/Prof Seet said that they were not cowed by its bad rap – rather, they were “emboldened”.
“Ultimately, our motivation to conduct the study was really to help the migrant workers, who were at the time facing great difficulties in both their mental and physical health,” he said.
Although the project initially seemed like “mission impossible”, it ultimately succeeded because of the efforts of the many volunteers within NUHS, who stepped up to offer their time and talents to the study.
“This was a ground-up, crowd-sourced project,” said A/Prof Seet.
“We set out to do this work, regardless of all the bad publicity and the lack of resources. And I’m very glad we did it.”
In consultation with A/Prof Raymond Seet, Senior Consultant, Division of Neurology, NUH; Professor Paul Tambyah, Senior Consultant, Division of Infectious Diseases, NUH.